Lunit AI Biomarker PD-L1 Demonstrates Clinical Efficacy as Diagnostic Aid in Cancer Treatment–Published in the European Journal of Cancer
May 19, 2022 - Lunit MediaStudy findings show that Lunit’s AI-powered PD-L1 TPS analyzer minimizes interpretation discrepancy among pathologists
AI-assisted PD-L1 TPS reading using Lunit SCOPE PD-L1 leads to better prediction of therapeutic outcomes in non-small cell lung cancer patients
In April 2022, Lunit SCOPE PD-L1 TPS received the CE Mark, becoming the first Lunit SCOPE product to receive regulatory approval
[SEOUL, South Korea, May 19, 2022] A recent study revealed that with the assistance of AI, multiple pathologists showed improved consensus in analyzing PD-L1 tumor proportion scores, finding more patients eligible for immunotherapy. The study has been published on May 14 in the European Journal of Cancer (EJC).
Findings from the study demonstrate the clinical efficacy of Lunit SCOPE PD-L1, the company’s AI-powered programmed death-ligand 1 (PD-L1) tumor proportion score (TPS) analyzer, which recently received the CE mark for commercial use in the European market.
“While PD-L1 expression is the standard biomarker for advanced non-small cell lung cancer (NSCLC), manual evaluation of PD-L1 TPS by pathologists has practical limitations of interobserver bias and intensive labor,” said Kyunghyun Paeng, Chief Product Officer of Lunit. “This study explores the role of Lunit’s AI-powered TPS analyzer in minimizing interobserver variation as well as enhancing prediction of therapeutic response to ICI.”
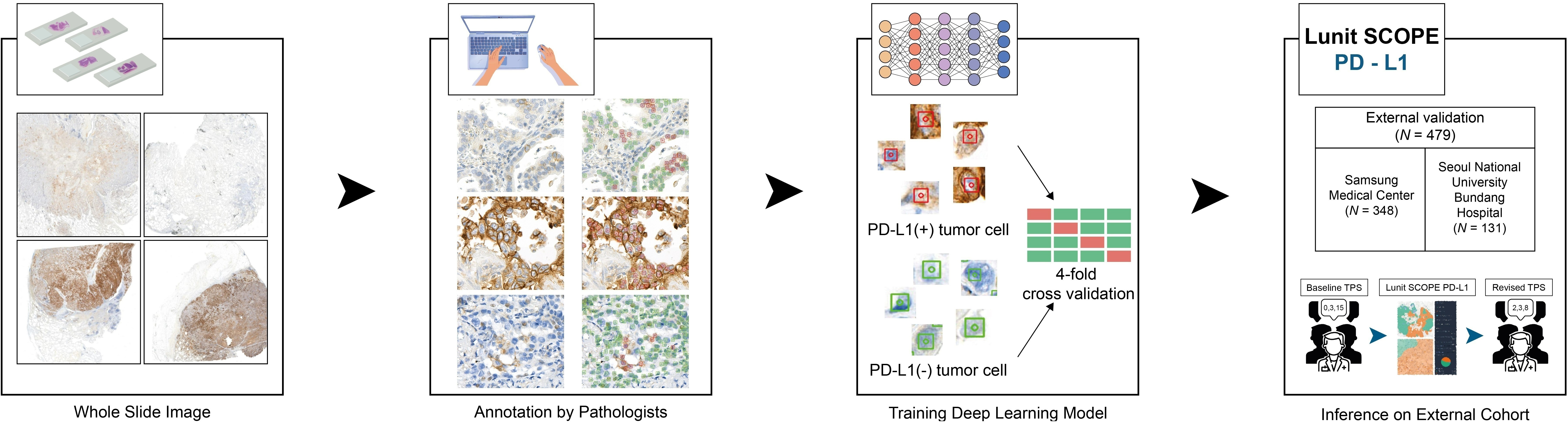
The development process of Lunit SCOPE PD-L1 ▲
In the study, three board-certified pathologists manually evaluated the PD-L1 TPS of 479 NSCLC data, concordantly scoring 81.4% of the cases. Following a revision of these evaluations with AI assistance, the consensus rate increased to 90.2%.
Furthermore, an analysis of ICI outcomes in 430 patients showed that AI-assisted evaluation led to more accurate survival predictions in terms of objective response rate, progression-free survival, and overall survival. In comparison to the outcomes of the baseline group with manual TPS evaluation, the subgroup after AI-assisted TPS revision showed a reduction in the hazard ratio for overall survival and progression-free survival upon ICI treatment.
The EJC is a peer-reviewed medical journal with an impact factor of 9.16 and is the official journal of the European Organisation for Research and Treatment of Cancer (EORTC) and the European Society of Breast Cancer Specialists (EUSOMA).
“This study is significant as it showed how AI can significantly make a difference in already established medical standards. Not only will our AI optimize pathologists' workflow, but it has the potential to find more patients eligible for anti-PD-L1 therapy, significantly increasing overall survival,” said Brandon Suh, CEO of Lunit.
Last month, Lunit SCOPE PD-L1 TPS received the CE Mark, becoming the first Lunit SCOPE product to receive regulatory approval. Lunit is currently working on launching the AI software in Europe within the second half of 2022.